Silver Spring, Md. – The Food and Drug Administration has unveiled a new proposal to help consumers make healthier dietary choices. The proposal requires front-of-package (FOP) nutrition labels on most packaged foods. Known as the "Nutrition Info box," the proposed label will prominently display information about a food's saturated fat, sodium, and added sugar content and categorize it as "Low," "Med," or "High."
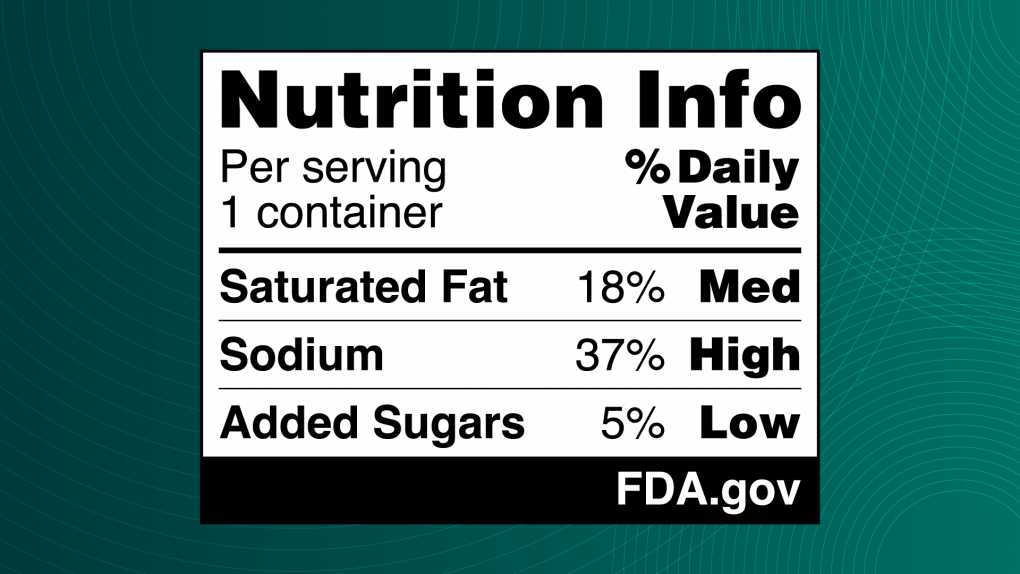
This initiative is a cornerstone of the FDA's efforts to combat diet-related chronic diseases, including heart disease, diabetes, and cancer. These diseases affect 60% of Americans and drive $4.5 trillion in annual healthcare costs. The FDA hopes to empower consumers to make informed decisions that align with healthy eating patterns by providing quick, at-a-glance nutrition information.
If finalized, the proposed rule would give large food manufacturers three years to comply, while smaller businesses with less than $10 million in annual sales would have four years.
FDA Commissioner Robert M. Califf, M.D., emphasized the importance of the proposed labeling in addressing the nation's chronic disease crisis. "Nearly everyone knows or cares for someone affected by a chronic disease linked to diet," said Califf. "This initiative makes it easier for consumers to glance, grab, and go, ensuring nutrition information is readily accessible as part of our public health mission."
The FOP Nutrition Info box complements the existing Nutrition Facts label by simplifying critical information and providing a visual reference for consumers on the levels of nutrients associated with chronic disease.
The proposed label is informed by extensive FDA research, including a 2023 experimental study of nearly 10,000 U.S. adults. The study evaluated consumer responses to different FOP label designs, finding that the black-and-white Nutrition Info scheme featuring percent Daily Values was most effective in helping participants quickly and accurately identify healthier options.
In addition to consumer education, FDA Deputy Commissioner for Human Foods Jim Jones noted the potential for the rule to incentivize manufacturers to reformulate products. "Food should promote wellness, not contribute to chronic disease," Jones stated. "By introducing front-of-package labeling, we hope to not only inform consumers but also encourage the food industry to offer healthier products."
The Nutrition Info box aligns with the White House National Strategy on Hunger, Nutrition, and Health, which aims to reduce diet-related diseases by 2030. It complements other FDA initiatives, including updated "healthy" claims, sodium reduction targets, and the development of a "healthy" symbol. Together, these efforts aim to create a healthier food supply and empower consumers to make better dietary choices.
Comments on the proposed rule can be submitted electronically to http://www.regulations.gov by May 16, 2025.








